What goes on inside our skulls? For the past decade, Dalhousie University physicist Tim Bardouille has been using neuroimaging to answer that question in his multidisciplinary Biosignal Lab, examining everything from stroke rehabilitation to how brains age.
So when a groundbreaking new technology for tracking brain activity became commercially available in 2018, Bardouille jumped on it. With funding from the CFI, he acquired the very first optically pumped magnetometer magnetoencephalography (OPM MEG) imaging system in Canada.
Like the multi-million-dollar conventional MEG systems found in a handful of hospitals across the country, OPM MEG creates a real-time picture of brain activity. But it’s far, far cheaper to buy and to operate. That makes powerful MEG neuroimaging technology available for a much broader range of clinical applications — and for research.
The OPM advantage
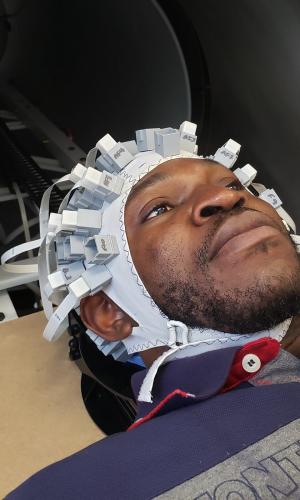
The principle behind MEG imaging is simple. Whenever neurons fire, they generate tiny magnetic fields. The challenge is detecting them. To do that, Bardouille explains, you need two things: highly sensitive sensors and a shield to block the Earth’s magnetic field and all the magnetic noise created by electronic devices nearby.
Conventional MEG systems use superconducting sensors that must be bathed in liquid helium and cooled to close to absolute zero (–269°C). That means the helmet containing the neuroimaging sensors is surrounded by massive cooling machinery, all housed in a magnetically shielded room.
By necessity, conventional systems use a one-size-fits-all helmet. “It works exceptionally well for people with average-sized adult heads,” Bardouille says. “But it works very poorly, for example, for children. It works very poorly for people who have a hard time staying still.”
Because OPM sensors use different technology, they don’t require cooling — and that’s a game-changer in all kinds of ways. Eliminating all the bulky equipment means the shield can be shrunk down to a two-metre-long cylinder equipped with a retractable patient bed, much like an MRI machine.
OPM sensors are also modular, so they can be attached to an adjustable cap that fits a wide range of patients. And while conventional sensors can’t sit directly on the scalp because they’re super-cooled, OPM sensors can. That allows them to pick up stronger signals, creating sharper images. “The literature suggests you get a four times signal boost,” says Bardouille. “That’s something that I’m trying to confirm with my system now.”
Building better brain imaging solutions
Bardouille predicts that OPM MEG will replace conventional MEG imaging in the next decade or two. But getting to that point will take a lot of work. With only a handful of OPM MEG systems in the world right now — Bardouille’s is still only one of two in Canada — it’s a very new technology that requires plenty of DIY effort to get up and running.
“You get sensors and a shield, and you have to make it into something that can measure brain activity. There’s a lot of missing bits there,” he explains.
Much of Bardouille’s work over the past six years has focussed on setting up the system, validating it and addressing technical challenges. For example, magnetic fields can warp inside the shield because it’s such a small space, so he’s been busy developing ways to compensate for that.
Unlocking clinical and research possibilities
At the same time, Bardouille has begun assessing the clinical potential of OPM MEG, starting with assessments for epilepsy surgery. PhD candidate Lindsey Power has been leading that project, leveraging her background in neuroscience and biomedical engineering.
If someone has a form of epilepsy that doesn’t respond to drugs, she explains, one treatment option is to remove the area of the brain where seizures originate. But before that happens, the surgeon needs to make sure it won’t affect crucial brain functions, like language.
That’s why Power has been validating the ability of OPM MEG to localize language centres in the brains of both epilepsy patients and a healthy control group. To do that, she takes scans of participants as they perform a series of auditory and visual tasks involved in recognizing and retrieving words.
So far, the results look promising. And that’s fuelling optimism that OPM MEG could eventually replace older imaging technologies like brain MRIs for a number of clinical applications — reducing wait times, lowering costs and improving patient outcomes.
OPM MEG also opens the door to all kinds of research possibilities, providing more brain-imaging power than the EEG machines typically found in universities. According to Power, being on the forefront of this disruptive technology has allowed Bardouille’s lab to advance their research in new directions and establish collaborations with other groups around the world exploring its uses.
“The fact that we were able to have the OPM in the lab and do such cutting-edge research and ask new questions, it’s really just broadened the scope of what we can do,” she says.

“This is an opportunity to really be at the forefront of a disruptive technology.”
— Tim Bardouille, Dalhousie University







